News
News selection
In the following you will find news from Polypharma Technologies & Sciences GmbH and the pharmaceutical industry.

Polypharma project planning for MENA pharma facilities
Core competencies of Polypharma include project planning, feasibility studies, plant construction, technology transfer, pharmaceutical development, and materials sourcing and supply. Its full service offerings can include all these elements to provide a total solution for pharmaceutical developers and producers that can also include processing registrations, with on-site services that include all necessary API-manufacturer documentation, such as GMP, CPP, manufacturing license, and Drug Master...
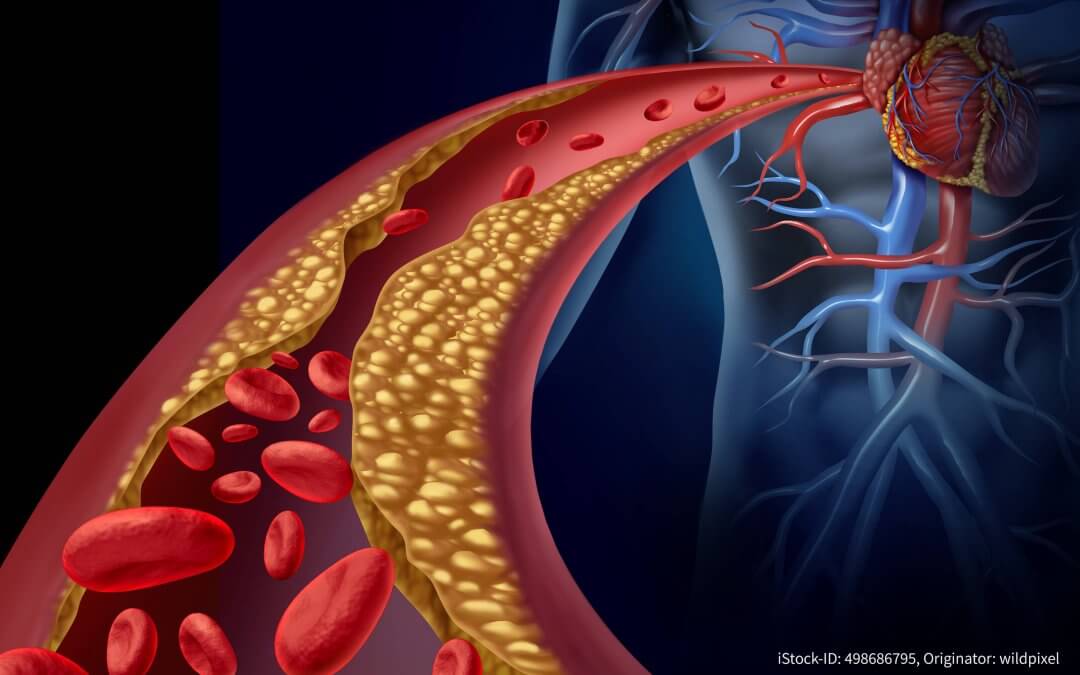
Polypharma marks World Cholesterol Day
Hamburg, Germany: Specialist pharmaceutical consultant and supplier Polypharma Technologies & Sciences GmbH (Polypharma) has been marking international Cholesterol Day. The substance cholesterol is involved in metabolic processes in the body, primarily in the metabolization of fat. The body covers its need for cholesterol by producing it in the liver and taking it from food. If excessive cholesterol circulates in the body, it accumulates in the vascular walls and facilitates the occurrence...

Polypharma celebrates 30th Anniversary
Single partner Polypharma offers a unique blend of services and skills that make it possible for it to act as a single partner accompanying local companies all the way from selection of target product through to actual receipt of raw materials for production. “Over the years we have developed more than 200 approved drug dossiers for our partners, meeting real local needs, along with tech transfer and up-scaling services to succeed with their own production,” noted Polypharma founder and...
Sodium hyaluronate 0.3% Serum
Polypharma has developed a Sodium hyaluronate 0.3% serum. Sodium hyaluronate, as hyaluronic acid, is a natural component of the skin and is used to smooth and tighten the skin, as well as to moisturise it.
Dossiers Betamethasone dipropionate 0.05% Lotion and Creme
The technical dossiers for Betamethasone dipropionate 0.05% lotion and Betamethasone dipropionate cream have been completed by Polypharma. This glucocorticoid is used to treat dermatitis, eczema and psoriasis.
Website relaunch
Our new website is online. Chlick for more: www.polypharma.de/en
API representation
Polypharma is now the representative of a Tunisian API manufacturer for: Donepezil Glimepiride (micronised) Bupivacaine Mepivacaine Ropivacaine Gliclazide Zolpidem Olanzapine If you are interested, send us a message by using our contact form or get in touch with us at: info[at]polypharma.de
Terbinafine hydrochloride 1% solution
Polypharma has developed a Terbinafine hydrochloride 1% spray solution for topical application. Terbinafine is an antifungal agent and the most commonly used treatment for skin and foot fungal infections.
Why Polypharma cannot recommend its partners to package purchased bulk medicines
Many new customers find it interesting to pack already manufactured goods as a first step before producing their own medicines. Here are the reasons why Polypharma cannot recommend buying bulk medicines: From a pharmaceutical point of view, production and packaging in a GMP-compliant production line is the highest priority for the quality of the pharmaceuticals Only with self-produced goods can you be 100% sure of the quality of the finished product If the manufacturing process and subsequent...
CPhI Worldwide 2020 in Milan
The CPhI worldwide 2020 in Milan in October 2020 was postponed to 2021 due to the COVID-19 pandemic. We would still like to keep in touch with you and invite you to participate in meetings via videoconference.https://www.cphi.com/europe/
Polypharma
Technologies & Sciences GmbH
Grosse Reichenstrasse 27
20457 Hamburg
Germany
+49 (0)40 37 480 3-0
info[at]polypharma.de